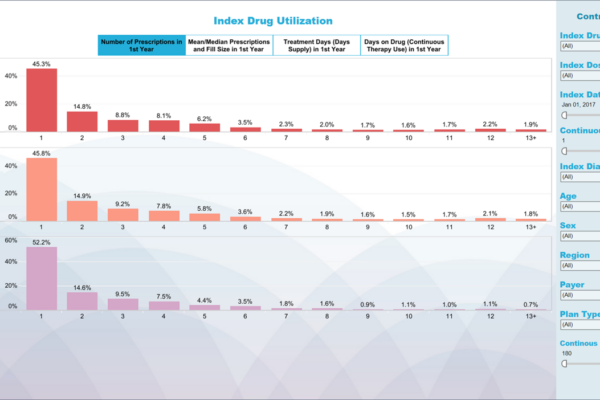
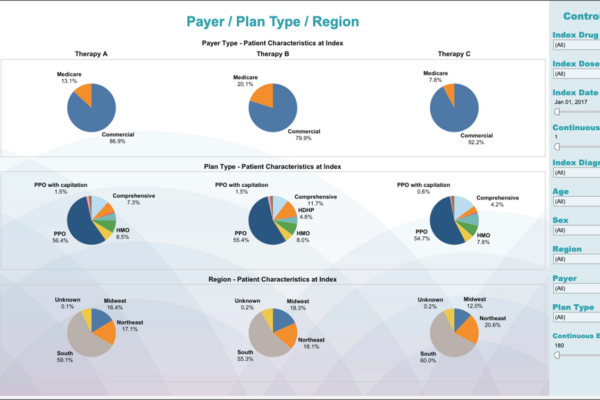
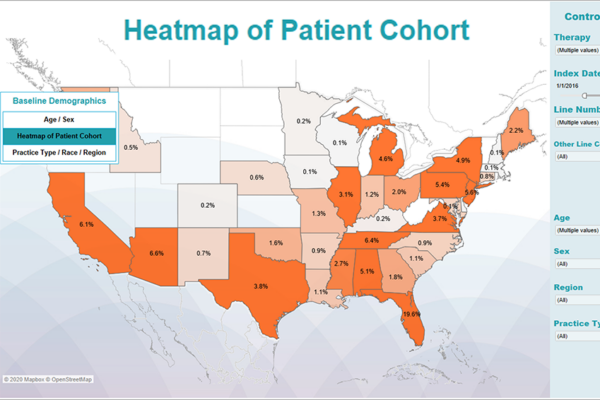
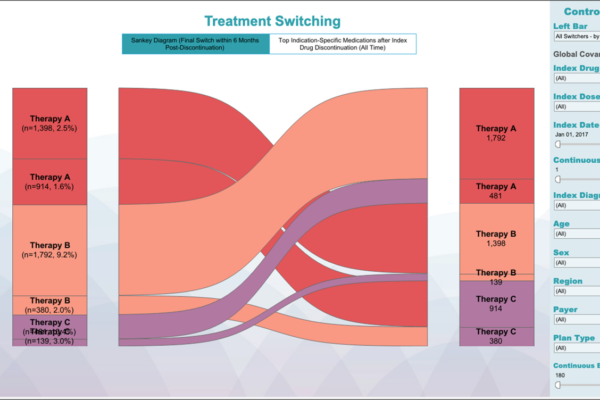
From early development to commercialization, you need the right data to support your evidence needs. Whereas some providers are limited by the datasets they use, our fit-for-purpose, data-agnostic real-world evidence expertise allows us to apply our knowledge of all relevant datasets to provide strategic advice and better demonstrate the value of your products.
For 16 years, we have: