Our Integrated Evidence Planning (IEP) Platforms offer an intuitive, fully customizable online solution to the complexity of evidence generation planning, management, alignment, and communication across critical stages of the product lifecycle, managing high volumes of data in such a way that HEOR product teams and stakeholders can quickly identify evidence and understand value messages.
IEP Platforms
Leverage technology to facilitate planning and optimize value demonstration
Collaborative online platforms that facilitate integrated evidence planning (IEP), provides optimal visibility to internal stakeholders, and ensures alignment across functional and geographic teams.

Why IEP Platforms?
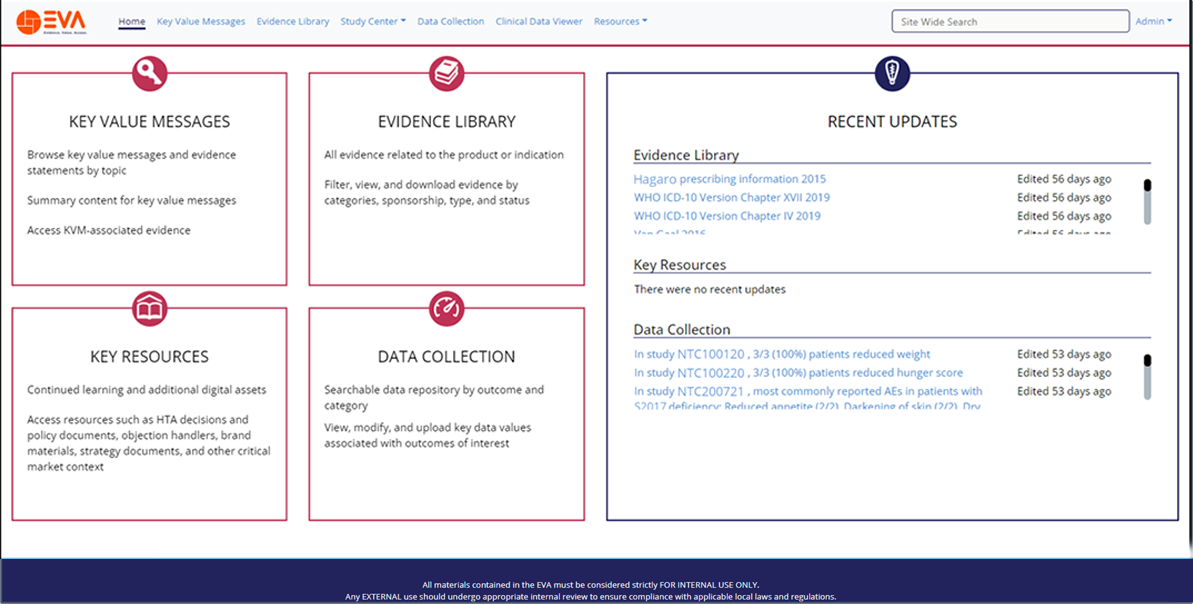
Manage your evidence generation through a single online hub
Find essential evidence and project updates quickly and easily
Share evidence and progress with colleagues and stakeholders
INTEGRATED EVIDENCE PLANNING
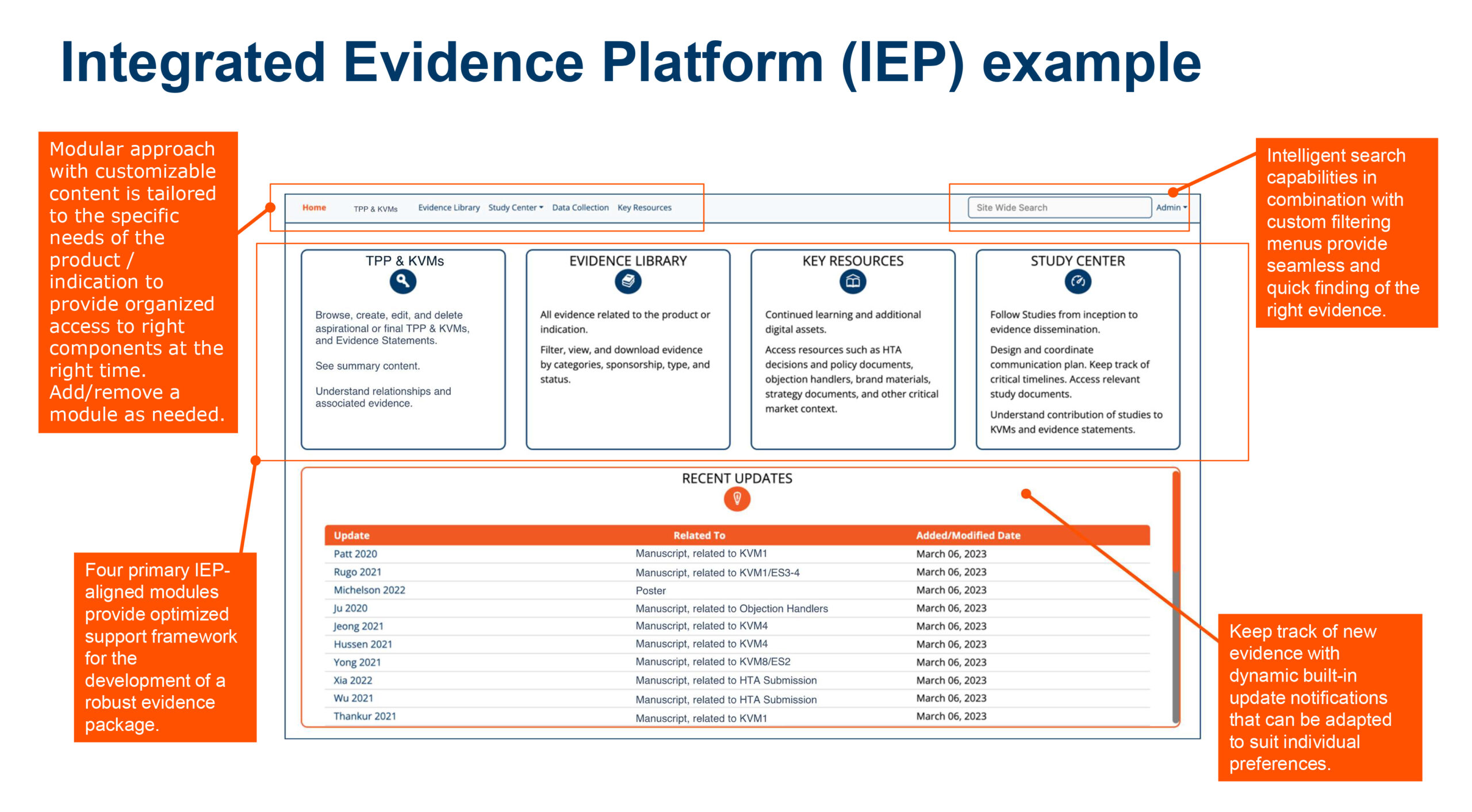
Enhancing evidence management and communication
- Centralized governance for evidence planning, generation and management
- Product evidence/value message alignment
- Tactical resource dissemination to team members and affiliates
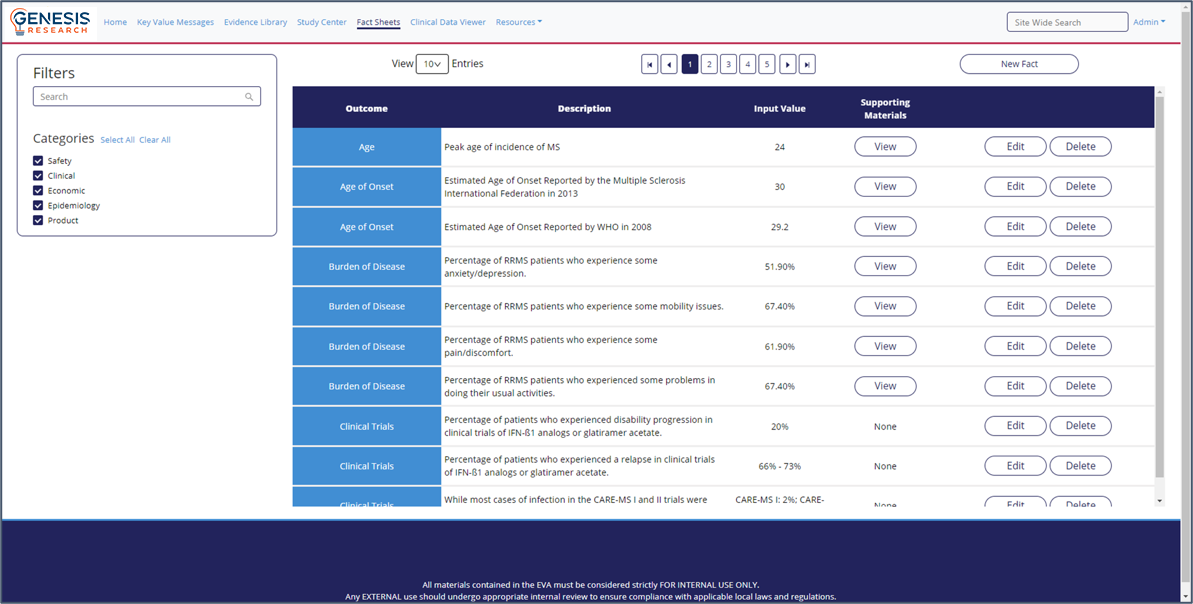
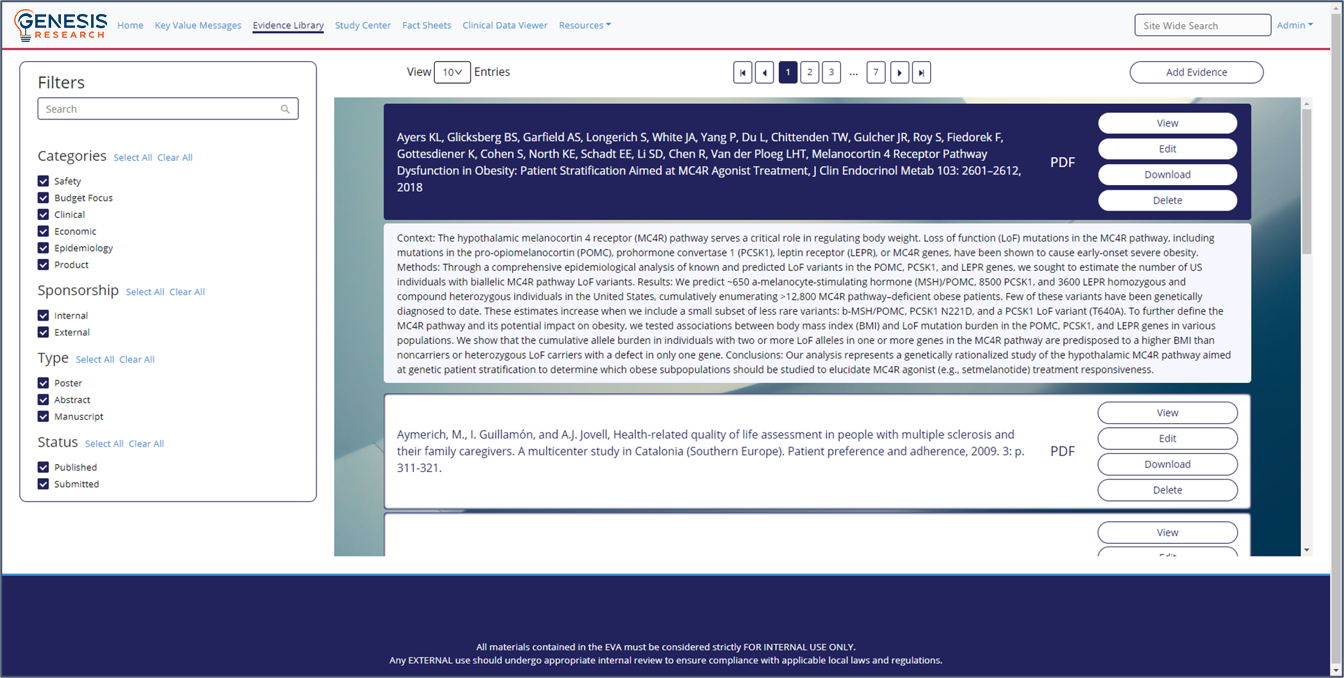
- Better understanding of the evidence base
- An intuitive interface and intelligent searches
- Repositories for IEP, documentation, evidence, and references.
Integrated Evidence Platform examples
Evidence strategy/IEP case study
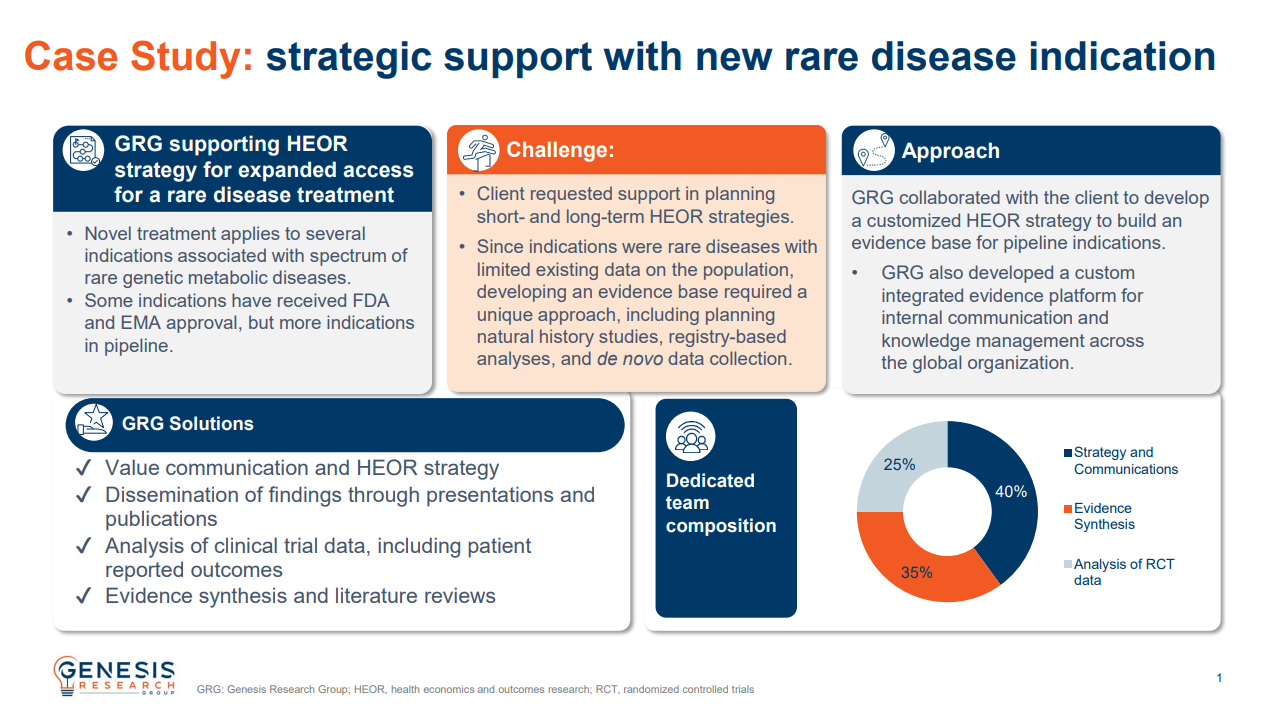
Strategic support with new rare disease indication
The client requested support in planning short- and long-term HEOR strategies.
Since indications were rare diseases with limited existing data on the population, developing an evidence base required a unique approach, including planning natural history studies, registry-based analyses, and de novo data collection.
We collaborated with the client to develop a customized strategy to build an evidence base for pipeline indications and a custom integrated evidence platform (IEP) that boosted internal communication and knowledge management across the global organization.
Our 8-step integrated evidence generation plan framework
Explore our wider integrated evidence generation planning offering: meeting the needs of multiple stakeholders across geographies and through the full product lifecycle.
Accelerate your insight generation
Our technology platforms play an integral role in our ability to provide life sciences companies with a better way to optimize insights and evidence.

Stakeholder insights when you need them
RPR is an agile, on-demand platform that leverages a network of 3,000+ payers and decision-makers in 65+ countries to provide clients with faster, higher-quality insights via direct automated stakeholder engagement which includes the ability to ask follow-up questions.

Integrated evidence planning
Tailored precisely to your needs, Integrated Evidence Planning (IEP) platforms provide an intuitive online solution to the complexity of evidence generation planning, management, alignment, and communication across critical stages of the product lifecycle.

AI-powered evidence synthesis
EVID AI is an AI-powered literature review platform that provides the world’s largest, up-to-date database of healthcare literature results and employs machine learning to extract and present the required data within structured evidence tables.
Headquarters:
HOBOKEN
111 River Street, Suite 1120
Hoboken, New Jersey 07030, US