Envision™ enables companies with development
stage oncology products to:
Envision™ Analogue
Forecasting oncology market access viability with payer-powered analogue analysis
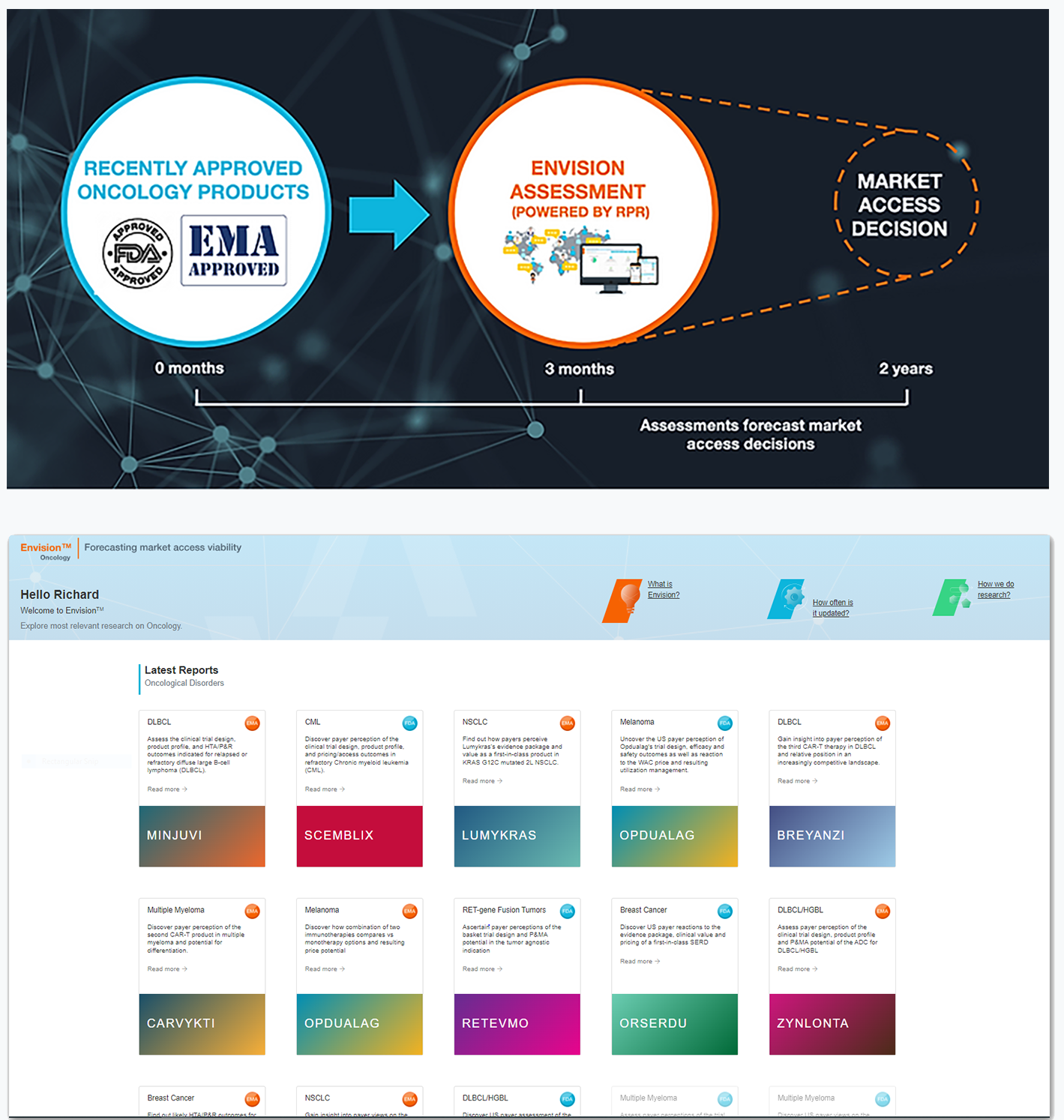
Powered by primary payer market research, Envision™ is an online platform that provides forward-looking evaluations and comprehensive strategic direction for market access viability after an oncology product has received positive EMA or FDA guidance.

Envision™ - leveraging the RPR™ payer network
3,500
65
24/7
Envision™ Analogue
Timely, rigorous and cost-effective analyses of recently reviewed oncology assets
- Understand early payer critiques of clinical data submitted to the FDA/EMA for registrations purposes, including that of competitors
- Learn from a reimbursement situation in which one tumour type has relevance for another
- Benchmark Target Product Profiles (TPPs)
- Analyze potential market access and pricing outcomes for competing products in the same indication
- Inform and improve clinical, market access and pricing strategies at the earliest possible stage
- Access a dynamic, growing library of analogue situations with relevance for your product.
Video
Envision: an introduction
Sample indications and products covered:
- NSCLC (Imfinzi + tremelimumab; Lumykras)
- Multiple myeloma (Talvey, Elrexfio, Carvykti)
- DLBCL (Tepkinly, Zynlonta, Breyanzi)
- Breast cancer (Orserdu, Enhertu)
- Melanoma (Opdualag)… plus more
Latest assessments:
- Talvey, Elrexfio (multiple myeloma)
- Tepkinly (DLBCL)
- Omjjara (myelofibrosis)
- Talzenna (prostate cancer)
Looking for a partner to help you predict the oncology landscape before your competitors?
Rapid stakeholder insights

Technology-infused research
Trust the voice of the stakeholder to test your evidence strategy and optimize market access
- Leverage our agile, tech-enabled RPR platform to develop robust market access strategies and fit-for-purpose evidence-generation plans.
- Secure critical payer, KOL, patient advocacy group, and policy maker insights across the product lifecycle.
- Engage with a global network of 3,500+ vetted stakeholders across 65+ countries to test your market access and evidence strategies as they evolve.

A global, vetted stakeholder network
3500+ experts across 65 countries are ready to provide the answers and clarifications you need
We search the globe for the most reputable experts before vetting them for suitability through a rigorous quality assurance and testing procedure. Each expert is tagged and classified according to their area of expertise and location, so that we can match the right experts to the right research objectives for our clients.
If you are an experienced healthcare decision-maker/stakeholder interested in helping to optimize patient outcomes, we invite you to consider sharing your expertise via our online survey platform, providing input when your schedule allows and being remunerated for your time.
Accelerate your insight generation
Our other equally powerful technology platforms play an integral role in our ability to provide life sciences companies with a better way to optimize insights and evidence.

Integrated evidence planning
Tailored precisely to your needs, Integrated Evidence Planning (IEP) platforms provide an intuitive online solution to the complexity of evidence generation planning, management, alignment, and communication across critical stages of the product lifecycle.

AI-powered evidence synthesis
EVID AI is an AI-powered literature review platform that provides the world’s largest, up-to-date database of healthcare literature results and employs machine learning to extract and present the required data within structured evidence tables.

Stakeholder insights when you need them
RPR is an agile, on-demand platform that leverages a network of 3,500+ stakeholders across 65+ countries to provide clients with faster, higher-quality insights via direct stakeholder engagement, including the ability to ask follow-up questions.
Headquarters:
HOBOKEN
111 River Street, Suite 1120
Hoboken, New Jersey 07030, US