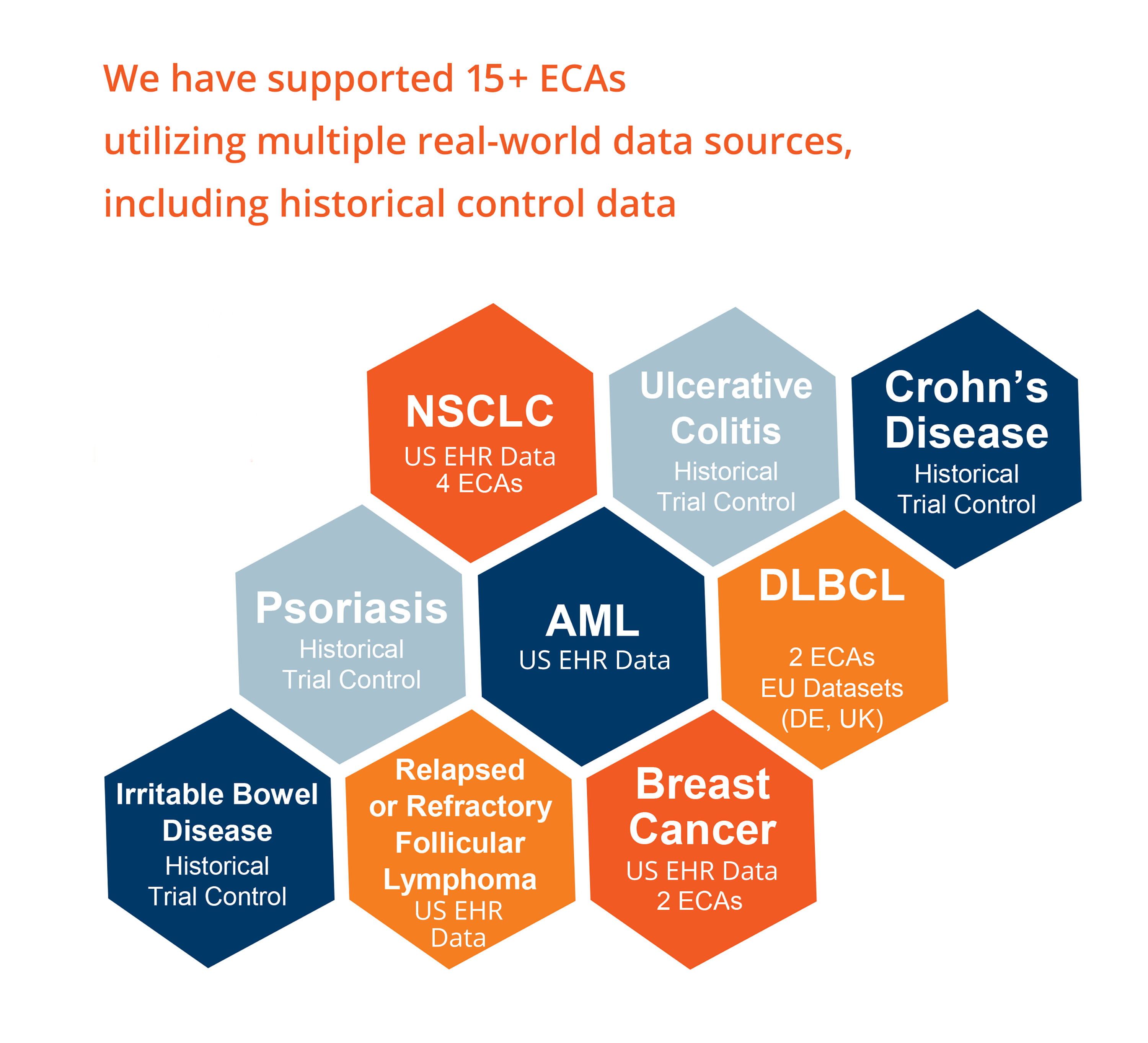
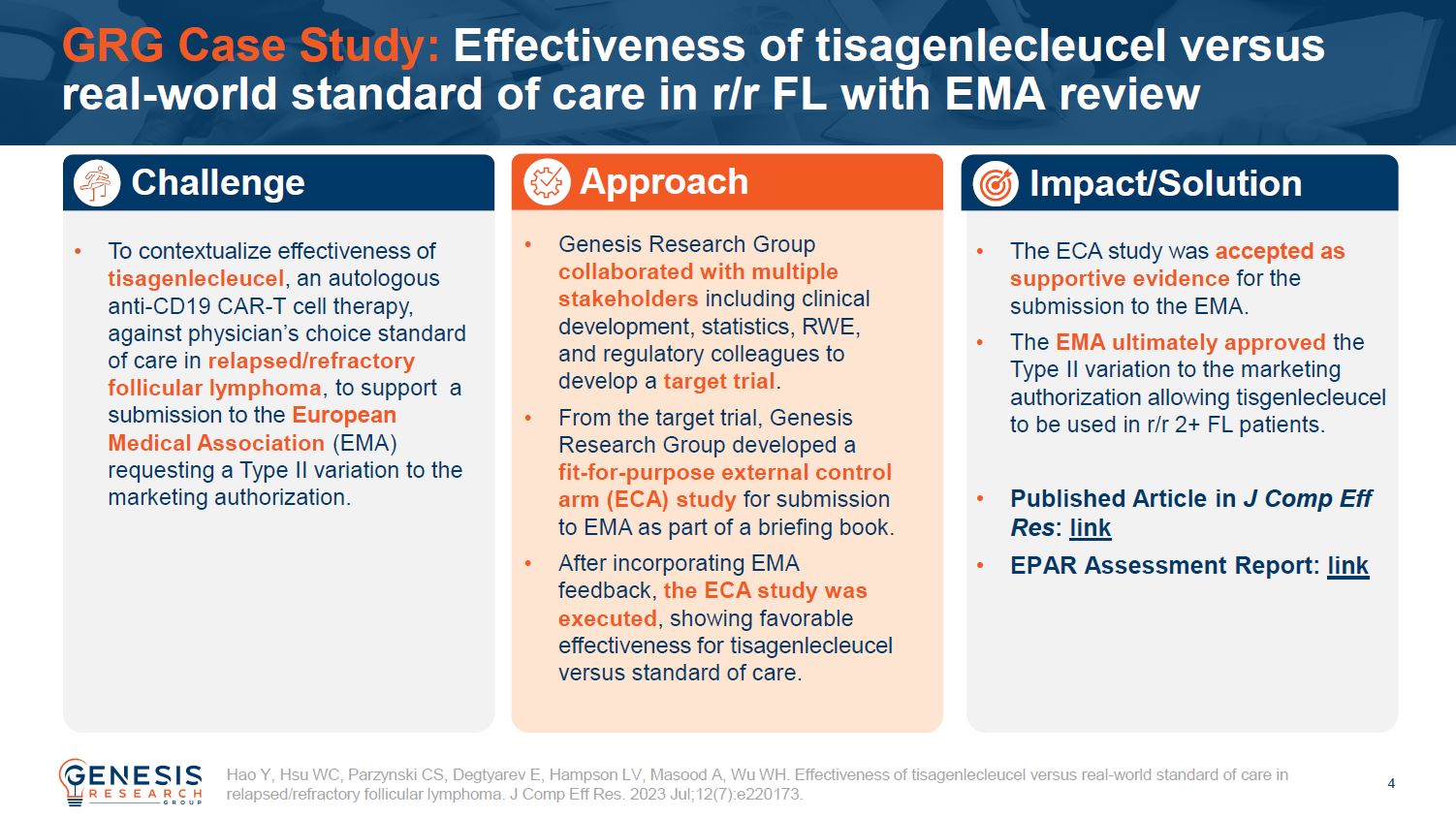
The effective use of external control arm studies remains a challenging proposition which requires significant expertise and experience to successfully navigate the data selection, design, analyses, and reporting required.
Why use an ECA?
To contextualize single-arm trial data (efficacy comparison, benchmark), when: